Abstract

Introduction: Acute lymphoblastic leukemia (ALL) represents approx. 5% of all newly diagnosed leukemias in patients (pts) between 55 and 70 years of age. Incorporation of novel antibody-based therapies in the first line induction therapy bears the potential of a significantly increased response rates and survival together with a reduction of treatment toxicity.
Methods: This open label phase II study (ClinicalTrials.gov, identifier: NCT03460522) of the German Multicenter Study Group on Adult Acute Lymphoblastic Leukemia was conducted at 13 centers. Pts aged >55 years with newly diagnosed Ph/BCR-ABL negative B-precursor ALL (at least CD20 positivity of leukemic blasts) were eligible. The 1st induction cycle consisted of InO 0.8mg/m2 on day1 and 0.5mg/m2 on d8 and d15 together with dexamethasone 10 mg/m2 (day7-8, day14-17) and one intrathecal injection (i.th.) of methotrexate (MTX), cytarabine (AraC) and dexamethasone (Dex). The 2nd and 3rd induction cycle consisted of InO 0.5mg/m2 on day 1, 8 and 15 plus i.th. MTX/AraC/Dex. Pts achieving a complete remission (CR) were offered to receive 5 conventional consolidation therapies (3 x ID-MTX/asparaginase; 2 x ID-AraC) and one reinduction therapy (idarubicine/ AraC/ cyclophosphamide / Dex.) in combination with rituximab (for CD20+ ALL), followed by a maintenance therapy with 6-MP/MTX. The primary endpoint was event free survival (EFS) at 12-months follow-up. An event was defined as any of the following: persisting bone marrow blasts after 2 cycles of InO, relapse or death. An event rate of ≤40% at 12 months follow-up was considered to qualify the experimental treatment as very promising for additional testing.
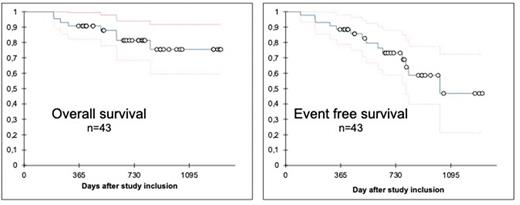
Results: 43 out of the 45 pts included were analyzed regarding the primary end point. Two pts were excluded per protocol from further analysis, as they received only one cycle of induction or withdraw consent. At diagnosis, median age was 64 years (range 56-80 years, 20 pts ≥65 years and 12 pts ≥70 years), median bone marrow blast count was 80% and median CD22 expression on leukemic blasts was 69% (range 21-99). 38 pts were diagnosed with a common- and 5 with a pro-B ALL. 42/43 pts completed all 3 cycles of induction therapy. All 43 pts achieved a complete remission (CR/CRi), mainly after the 1st induction. Median time between start of 1st induction to start 2nd induction, from 2nd to 3rd induction and from 3rd induction to 1st consolidation therapy was 21 days, 28 days and 30 days, respectively. Results of minimal residual disease (MRD), measured by real-time quantitative PCR, are available for all 43 pts, with 23/43 (53%) and 31/42 (74%) pts being MRD negative after 2nd and 3rd induction therapy, respectively. With a median follow-up of 697 days, the probability of OS at 1 and 2 years is 91% (95% C.I. 82-99%) and 81% (95% C.I. 69-94%), respectively. No patient died within the first 6 months after start of induction therapy. 5 events occurred within the first year after study inclusion, 3 deaths in CR and 2 relapsed ALL, resulting in event rate of 12% and a probability of EFS at 1 year (primary endpoint of the study) of 88% (95% CI 79-98). EFS at 2 years was 73% (95% CI 59-88). So far, 7 pts with persisting/reappearance of MRD (2 pts) or relapse (5 pts) were treated with blinatumomab. 9 pts received an aSCT (4 pts in CR1 and 5 pts >CR1), 6 of these pts are alive in CR. Regarding adverse events (AEs) during induction cycles 1, 2 and 3, most common AEs ≥CTC grade 3 reported were leukocytopenia (in 74%, 19% and 2% of all cases, respectively), anemia (37%, 5%, 0%), thrombocytopenia (49%, 7%, 2%) and elevation of liver enzymes (14%, 5%, 0%). One patient was reported with suspected veno occlusive disease (after 2nd induction therapy). Classical factors which may be associated with inferior survival outcomes like age, diagnosis of pro-B ALL, disease burden at diagnosis, co-morbidity or no MRD negative CR after induction had no significant impact on OS or EFS.
Conclusion: Three cycles of InO as induction therapy for the treatment of older pts with de novo acute B-lymphoblastic leukemia resulted in a very high remission rate. Given that our study met the primary end point (predefined event rate at 1 year ≤40%, observed event rate 12%), these promising study results could be a rationale for integrating InO induction therapy in future treatment recommendations. Ideally, this novel induction therapy should be evaluated in prospective randomized trials.
Disclosures
Stelljes:Pfizer: Consultancy, Honoraria, Research Funding; Amgen: Consultancy; Novartis: Consultancy, Honoraria; Medac: Honoraria; MSD Sharp & Dohme: Consultancy; Kite: Consultancy, Honoraria; Jazz: Honoraria; MSD: Consultancy, Honoraria. Alakel:Pfizer: Consultancy, Honoraria. Wäsch:Pfizer: Consultancy, Honoraria. Nachtkamp:Jazz: Honoraria; BSH medical: Honoraria. Haenel:Takeda: Consultancy, Honoraria; GSK: Consultancy, Honoraria; Gilead: Consultancy, Honoraria; Celgene: Consultancy, Honoraria; Bayer: Consultancy, Honoraria; Amgen: Consultancy, Honoraria; Pfizer: Honoraria; Novartis: Consultancy, Honoraria; Roche: Consultancy, Honoraria; JAZZ: Consultancy, Honoraria. Wethmar:Pfizer: Honoraria. Sauer:Pfizer: Honoraria; Abbvie: Honoraria, Membership on an entity's Board of Directors or advisory committees; Jazz: Honoraria; Amgen: Membership on an entity's Board of Directors or advisory committees; Astellas: Membership on an entity's Board of Directors or advisory committees; BMS: Membership on an entity's Board of Directors or advisory committees; Gilead: Membership on an entity's Board of Directors or advisory committees; Takeda: Membership on an entity's Board of Directors or advisory committees; Ridgeline Discoveries: Membership on an entity's Board of Directors or advisory committees. Lenz:Morphosys: Consultancy, Honoraria, Research Funding, Speakers Bureau; Genmab: Consultancy, Honoraria; BMS: Consultancy, Honoraria, Speakers Bureau; Bayer: Consultancy, Honoraria, Research Funding; Novartis: Consultancy, Honoraria, Speakers Bureau; AstraZeneca: Consultancy, Honoraria, Research Funding, Speakers Bureau; Abbvie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Incyte: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Karyopharm: Consultancy, Honoraria; Constellation: Consultancy, Honoraria; ADC Therapeutics: Consultancy, Honoraria; Miltenyi: Consultancy, Honoraria; Takeda: Honoraria, Speakers Bureau; Janssen: Consultancy, Honoraria, Research Funding, Speakers Bureau; Gilead: Consultancy, Honoraria, Research Funding, Speakers Bureau; Roche: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; University Hospital Münster: Current Employment. Goekbuget:Erytech: Other: Advisory board; Cellestia: Other: Advisory board; AstraZeneca: Other: Invited talk for company-sponsored symposia (with honoraria); AbbVie: Other: Research funding (institution); Gilead/Kite: Other: Research funding (institution); invited talk; advisory board; Incyte: Other: Research funding (institution); advisory board; Jazz Pharmaceuticals: Other: Research funding (institution); advisory board; Novartis: Other: Research funding (institution); advisory board; Servier: Consultancy, Other: Research funding (institution); invited speaker; advisory board; Pfizer: Consultancy, Other: Research funding (institution); invited speaker; advisory board; Amgen: Consultancy, Other: Research funding (institution); invited speaker; advisory board; MorphoSys: Other: Advisory board; Clinigen: Other: Advisory board.
OffLabel Disclosure:
Inotuzumab is not approved for 1st line therapy
Author notes
 This icon denotes a clinically relevant abstract
This icon denotes a clinically relevant abstract
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal